I have found it non linear. In contrast the electrochemical response of nanostructured electrodes was strongly dependent on the scan rate.

All Printed Magnetically Self Healing Electrochemical Devices
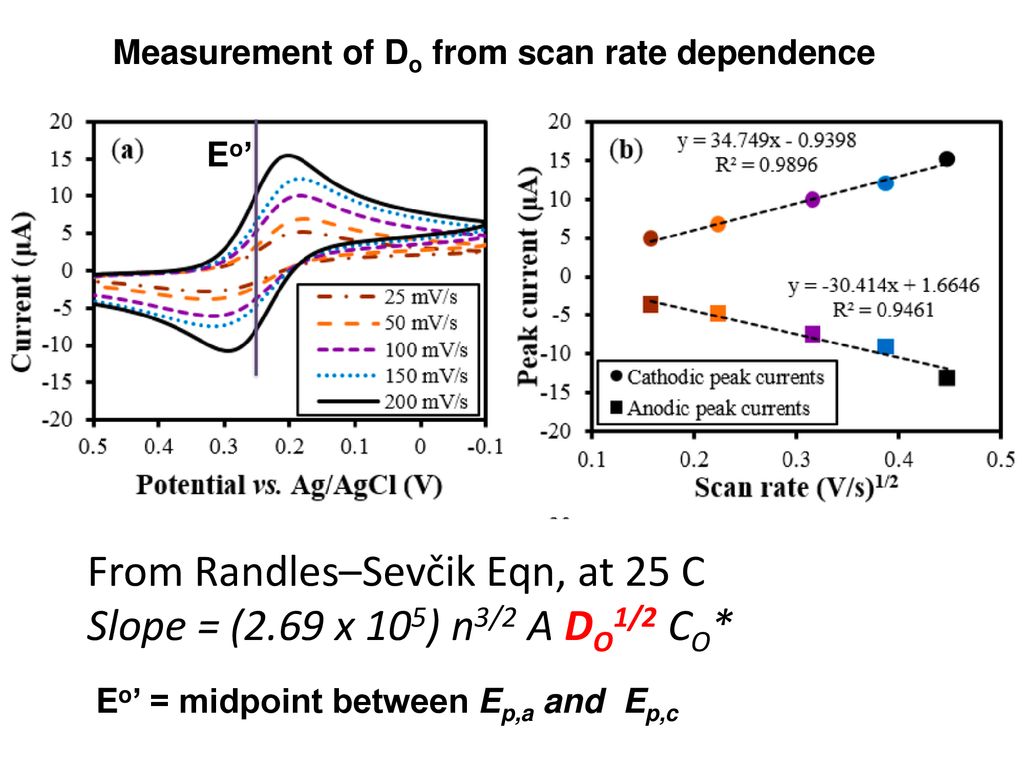
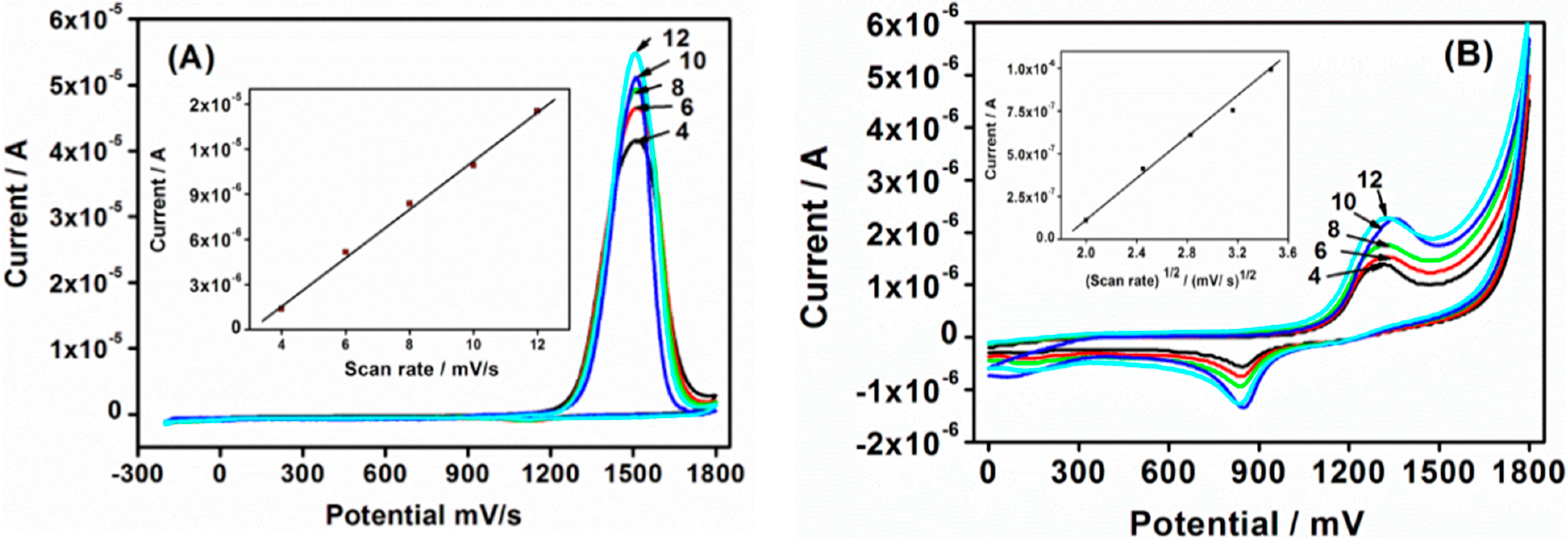
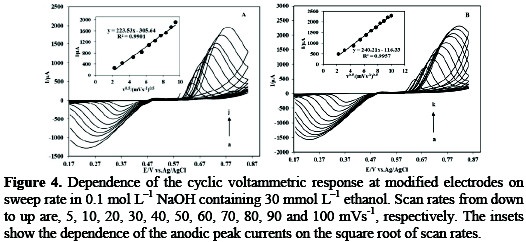
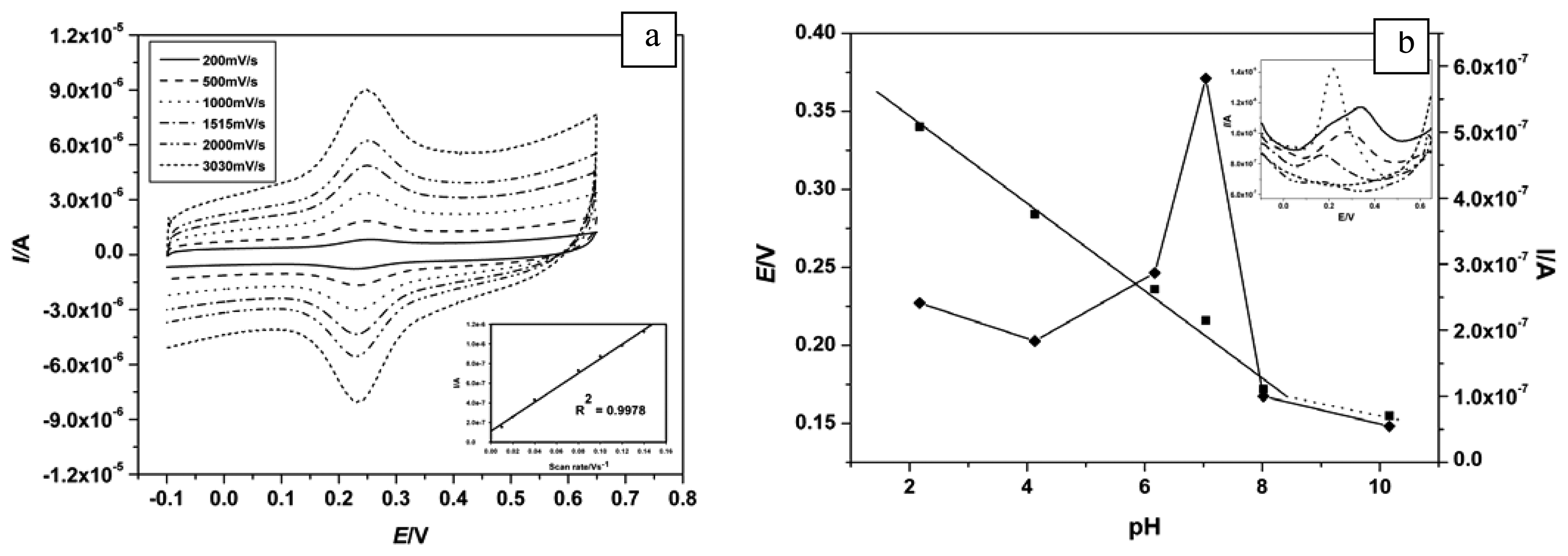
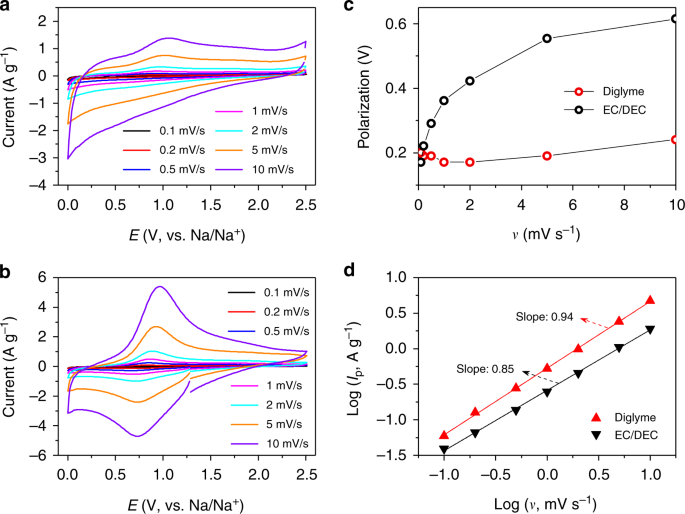
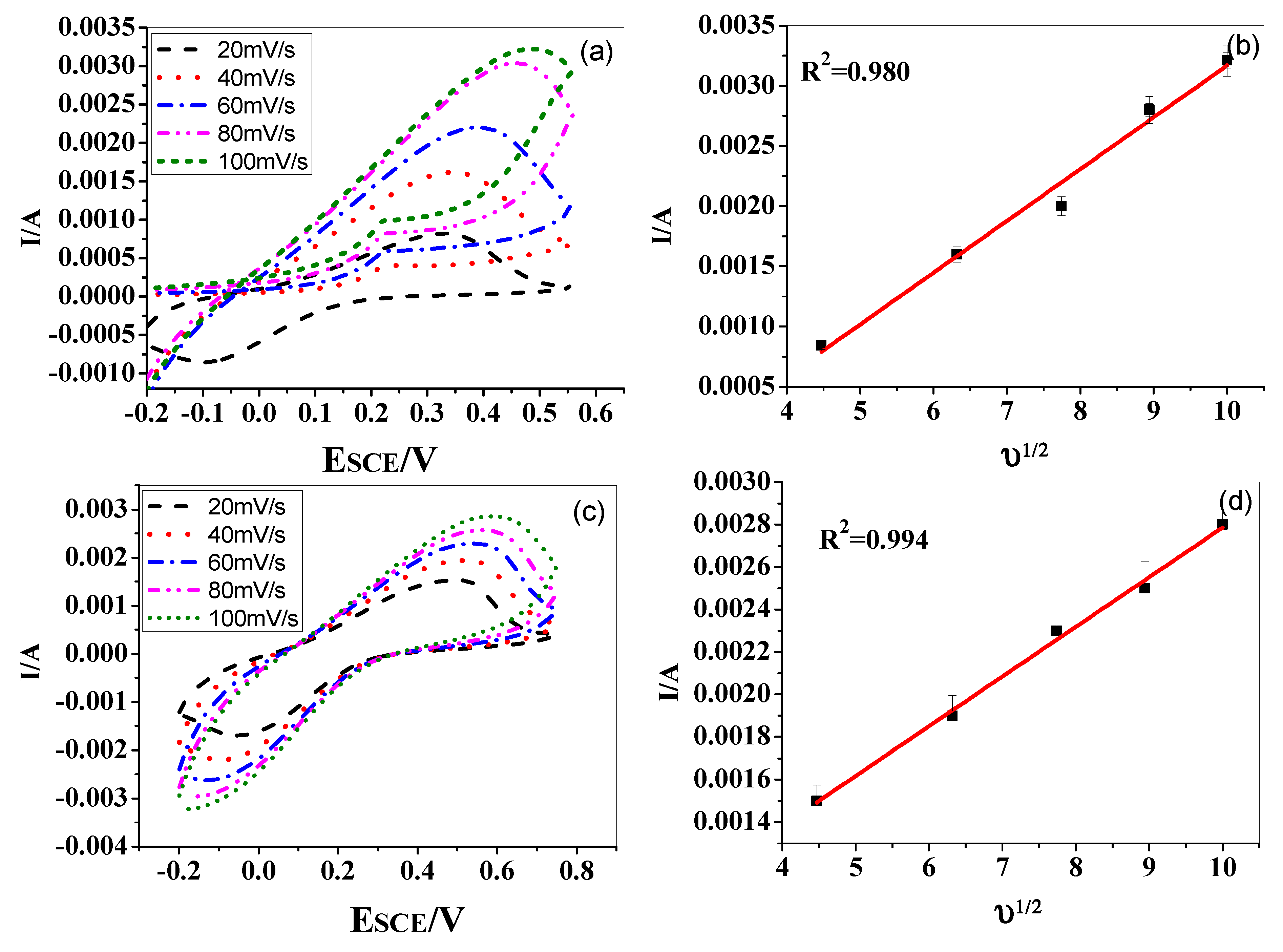
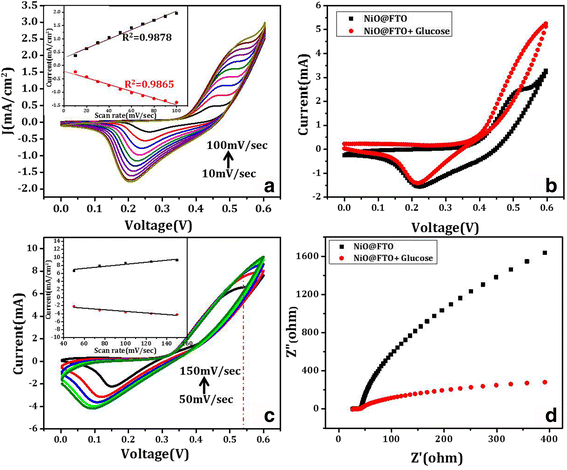
Cv scan rate dependence. In a cyclic voltammetry experiment the working electrode potential is ramped linearly versus time. The diffusion coefficient d for lithium ion intercalation into the wo 3 film. So according to the above equation and to keep the same charge value the current should be increased thats way the peak current is. As the current is proportional to the flux towards the electrode the magnitude of the current will be lower at slow scan rates and higher at high rates. Cyclic voltammetry normalized by scan rate. In order to assess the effect of the scan rate on the li ion diffusion coefficient as calculated from cyclic voltammetry cv a series of cv experiments at various rates namely 2 5 10 20 and 50 mvs has been carried out for the same wo 3 film prepared by electron gun deposition at room temperature.
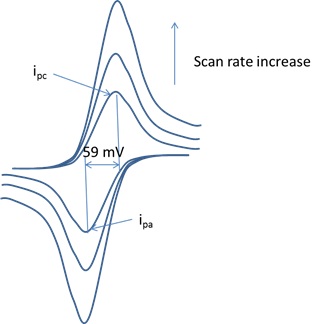
When the scan rate is increased the time will be decreased. Unlike in linear sweep voltammetry after the set potential is reached in a cv experiment the working electrodes potential is ramped in the opposite direction to return to the initial potential. Cyclic voltammetry cv is a type of potentiodynamic electrochemical measurement. However at the slower scan rate the peak on the reverse scan disappears. Scan rate dependence of cv and its peak current ip of a reversible reaction system. I have recorded the cvs at different scan rate 100 1000 mv and plotted the graphs for peak current vs square root of scan rate.
This highlights an important point when examining lsv and cyclic voltammograms although there is no time axis on the graph the voltage scan rate and therefore the time taken to record the voltammogram do strongly effect the behaviour seen. A second capacitor was used to show cvs scan rate dependence. The expected characteristics of the cv were only obtained with a very slow scan rate of 2 mv s 1. Rates are given by kredox z exp µ gredox kbt 16 if we plot a series of the free energy profiles as a function of voltage the free energy of r will be invariant with voltage whereas the right handside o e shows a strong dependence. This is a distinct advantage of cyclic voltammetry over other voltammetric techniques. Scan limits were 00 and 27 v.
In the case of polycrystalline electrodes the buffer capacity was kept close to the equilibrium state over the whole scan rate range. O ne r 2 and there is no homogenous reaction and surface adsorption of any reactant and product. The capacitor was held at 00 v for 10 min between scans. If the kinetic parameter electron transfer rate k 0 is sufficiently fast for a reaction. The illustration below for example shows the cyclic voltammogram for the same redox couple at both a faster a and a slower b scan rate. At the faster scan rate we see two peaks.
Voltammograms were recorded at scan rates of 316 10 316 100 and 316 mvs.